Difference between revisions of "Neuroscience"
| Line 22: | Line 22: | ||
:[http://www.qiagen.com/us/products/genes%20and%20pathways/pathway%20details.aspx?pwid=55 BDNF Pathway] | :[http://www.qiagen.com/us/products/genes%20and%20pathways/pathway%20details.aspx?pwid=55 BDNF Pathway] | ||
| − | [www.ncbi.nlm.nih.gov/pubmed/21613508 Functional role of neurotrophin-3 in synapse regeneration by spiral ganglion neurons on inner hair cells after excitotoxic trauma in vitro] <-- NT3 vital for restoring ear-hair-cell-neurons (TINNITUS?). | + | [http://www.ncbi.nlm.nih.gov/pubmed/21613508 Functional role of neurotrophin-3 in synapse regeneration by spiral ganglion neurons on inner hair cells after excitotoxic trauma in vitro] <-- NT3 vital for restoring ear-hair-cell-neurons (TINNITUS?). |
---- | ---- | ||
Revision as of 18:50, 13 September 2014
TODO:
http://www.sabiosciences.com/rt_pcr_product/HTML/PAHS-126Z.html ? http://journal.frontiersin.org/Journal/10.3389/neuro.02.001.2010/full ?
Dynamic epigenetic regulation in neurons <-- HDACi VALPROATE? !!!
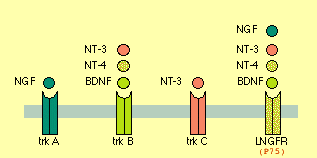
- Neurotrophin = {NGF, BNDF, NT3, NT4}
- Neurotrophic Factor = Neurotrophin + {GDNF, CNTF}.
NGF -- promotes Axon growth (branching and a bit of elongation)
BNDF -- CNS&PNS, supports survival of existing neurons, growth & diff'n of new neurons & synapses. DiHexa = 10M * BNDF. Binds to TrkB and P75 (a.k.a. L-NGFr) receptors.
Functional role of neurotrophin-3 in synapse regeneration by spiral ganglion neurons on inner hair cells after excitotoxic trauma in vitro <-- NT3 vital for restoring ear-hair-cell-neurons (TINNITUS?).
GSK3 -- can't quite fit this anywhere else, maybe relevant maybe not
Role of GSK3 signaling in neuronal morphogenesis
GSK3 signalling in neural development
Roles of PI3K/AKT/GSK3/mTOR Pathway in Cell Signaling of Mental Illnesses
Axon Growth-Stimulus Package Includes Local Translation
- Translation of localized mRNAs is an important mechanism for controlling spatially discrete cellular processes. The polarity protein Par-3 is locally translated in axons in response to factors such as NGF and netrin-1, and this increased expression is necessary for factor-stimulated axonal outgrowth.
- When and NGF bead lands on an axon, a new growth cone is induced at the contact site, and a branch sprouts from the axon.
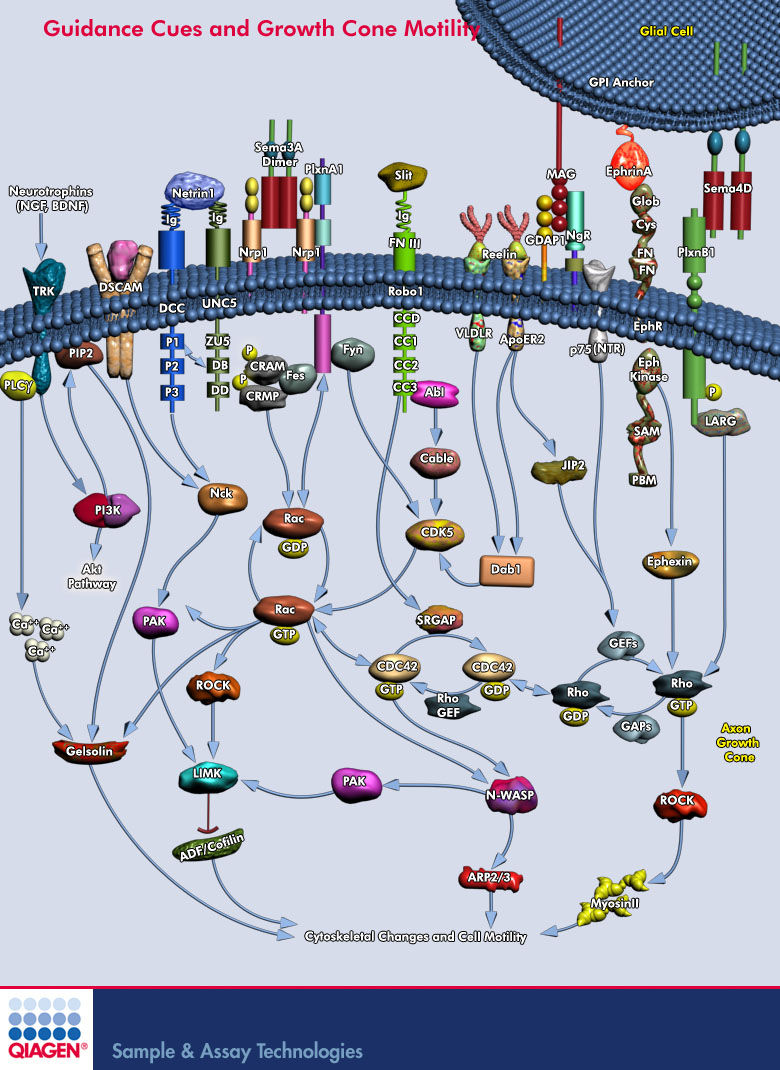
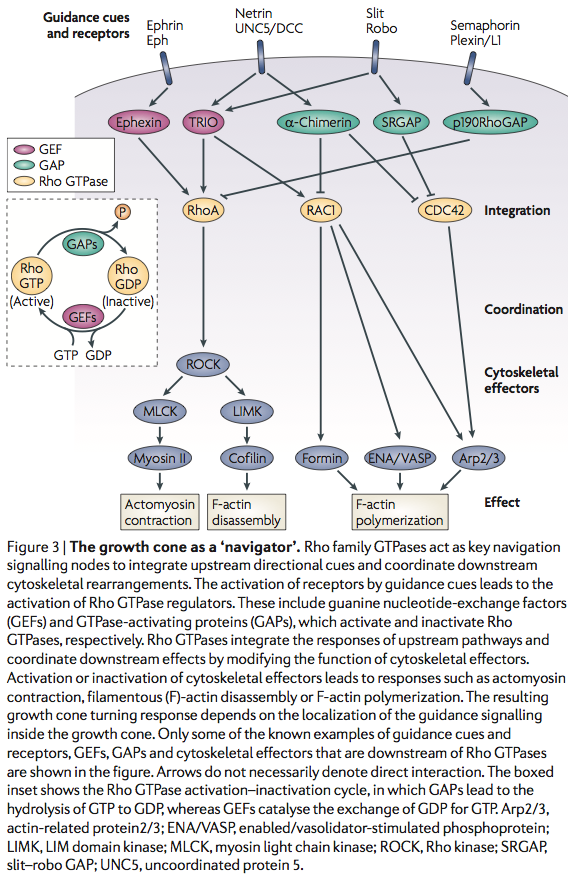
Lamellipodium <-- 'Rac promotes lamellipodia while cdc42 promotes filopodia' (filopodia are the fingers, the probes on the growthcone and lamellipodium is the webbing)
http://en.wikipedia.org/wiki/Semaphorin <-- Semaphorins are a class of secreted and membrane proteins that act as axonal growth cone guidance molecules. [...] The main receptors for semaphorins are plexins, which have established roles in regulating Rho-family GTPases. Recent work shows that plexins can also influence R-Ras, which, in turn, can regulate integrins.
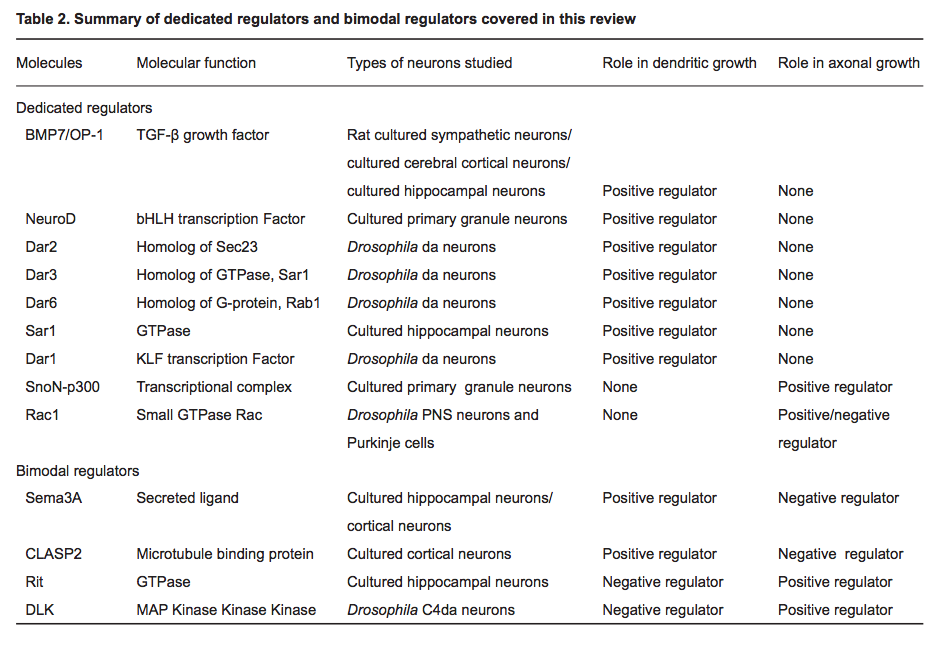
(2014) Regulatory mechanisms underlying the differential growth of dendrites and axons *
- "Recent studies have uncovered two distinct types of regulatory mechanisms that differentiate dendritic and axonal growth: dedicated mechanisms and bimodal mechanisms. Dedicated mechanisms regulate either dendrite- specific or axon-specific growth; in contrast, bimodal mechanisms direct dendritic and axonal development in opposite manners."
The Novel GTPase Rit Differentially Regulates Axonal and Dendritic Growth
Sema3A is where?
Sema3A -> CRMP -> Rac/GDP -> Rac/GTP -> CDC42 -> Rho -> ROCK -> myosinll
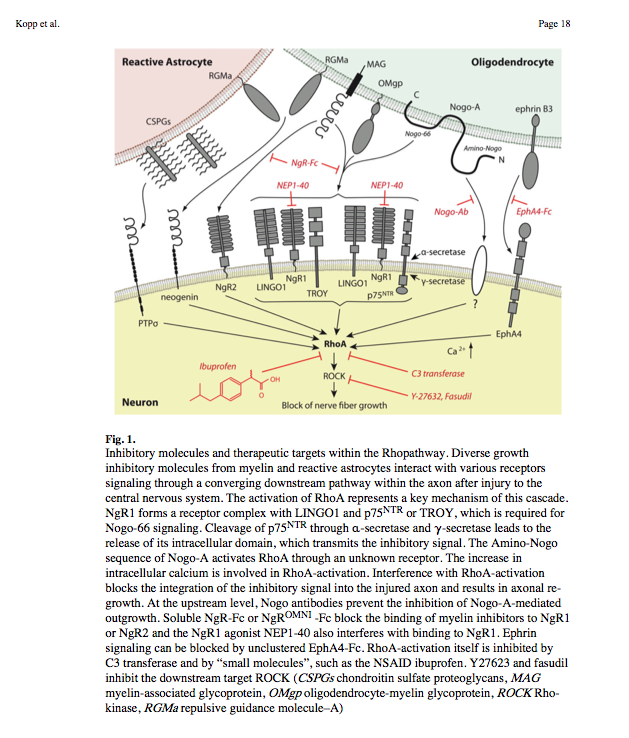
(2010) Deciphering Proteins and their Functions in the Regenerating Retina
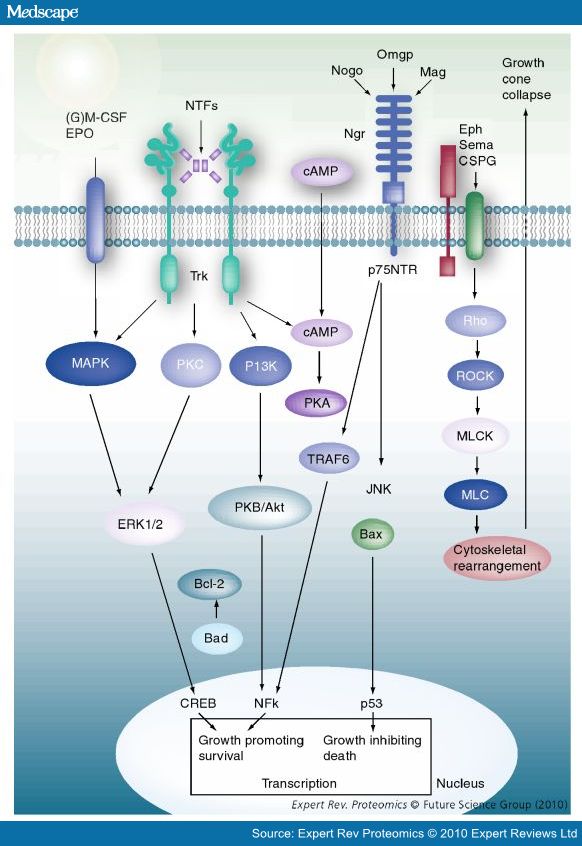
- Nogo-A, -B and -C all contain a 66-amino acid extracellular domain (Nogo-66) that alone can inhibit neurite outgrowth and induce growth-cone collapse.
- Remarkably, a single neuronal protein (NgR), binds Nogo, MAG and OMgp.[101] NgR is thought to form a complex with its coreceptors (p75NTR, TROY, a member of the TNF receptor family, and Lingo-1), thereby transducing a myelin/NgR signal as the inhibitory signal to the cell interior[95] and potentially activating the small GTPase RhoA (Figure 3B). Another myelin-associated neurite outgrowth inhibitor, the RGM, which activates the Rho/ROCK signaling cascade independently of the NgR pathway, has recently been discovered. Consistent with these findings, deleting oligodendrocytes, myelin or Schwann cell-derived factor-induced cleavage of NgR and Nogo-A, and inactivation of p75NTR [105] enhances the regeneration of descending visual pathways. Consistent with their inhibitory effects on neurite outgrowth, approaches counteracting NgR, Nogo-A or RhoA have been shown to significantly enhance long-distance axon regeneration in animal models of acute retinal injury.
- ^ need to check up on RGM! Looks as though disabling NgR is not sufficient. also need to disable RGM as either one can activate Rho -> ROCK.
- (Wait: The therapeutic effects of Rho-ROCK inhibitors on CNS disorders <-- says 'In the adult CNS, injured axons regenerate poorly due to the presence of myelin-associated axonal growth inhibitors such as myelin-associated glycoprotein (MAG), Nogo, oligodendrocyte-myelin glycoprotein (OMgp), and the recently identified repulsive guidance molecule (RGM). The effects of these inhibitors are reversed by blockade of the Rho-ROCK pathway in vitro, and the inhibition of this pathway promotes axonal regeneration and functional recovery in the injured CNS in vivo.')
So how about directly disable Rho using Ibuprofen:
Small-molecule-induced Rho-inhibition: NSAIDs after spinal cord injury
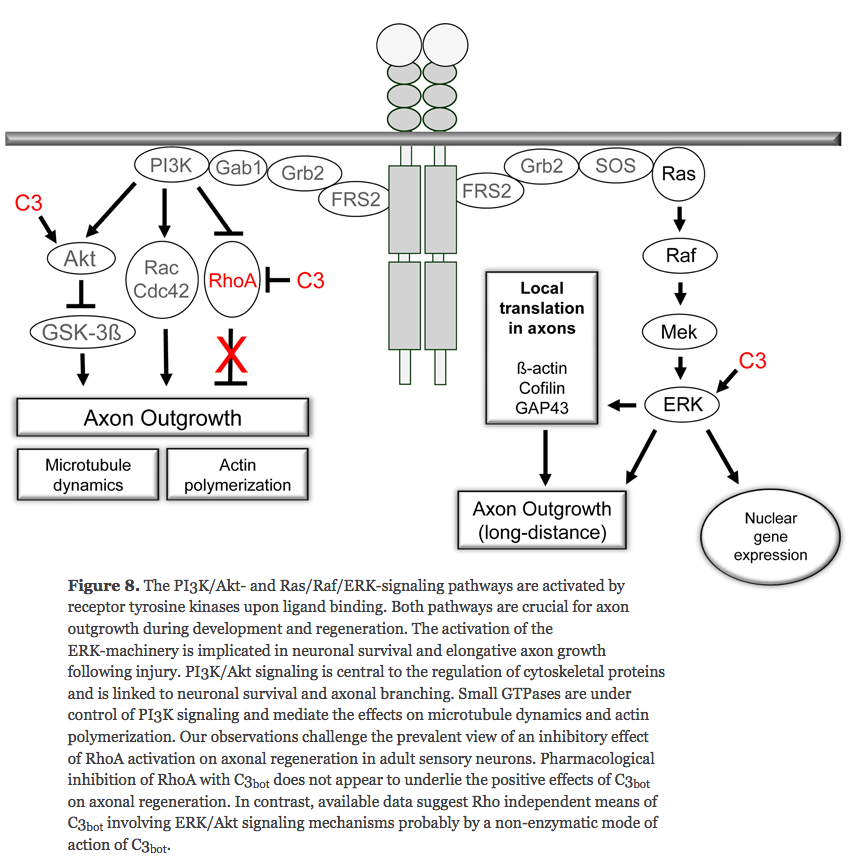
(2005) Local translation of RhoA regulates growth cone collapse
- Neuronal development requires highly coordinated regulation of the cytoskeleton within the developing axon. This dynamic regulation manifests itself in axonal branching, turning and pathfinding, presynaptic differentiation, and growth cone collapse and extension. Semaphorin 3A (Sema3A), a secreted guidance cue that primarily functions to repel axons from inappropriate targets, induces cytoskeletal rearrangements that result in growth cone collapse1. These effects require intra-axonal messenger RNA translation. Here we show that transcripts for RhoA, a small guanosine triphosphatase (GTPase) that regulates the actin cytoskeleton, are localized to developing axons and growth cones, and this localization is mediated by an axonal targeting element located in the RhoA 3' untranslated region (UTR). Sema3A induces intra-axonal translation of RhoA mRNA, and this local translation of RhoA is necessary and sufficient for Sema3A-mediated growth cone collapse. These studies indicate that local RhoA translation regulates the neuronal cytoskeleton and identify a new mechanism for the regulation of RhoA signalling.
Intra-Axonal Translation of RhoA Promotes Axon GrowthInhibition by CSPG
- RhoA transcripts have been detected in axons of embryonic dorsal root ganglia (DRG), retinal ganglion, and cortical and hippocampal neurons, and are locally translated in DRG axons in response to the inhibitory guidance cue Semaphorin 3A (Sema3A) and mediate growth cone collapse (Wu et al., 2005).
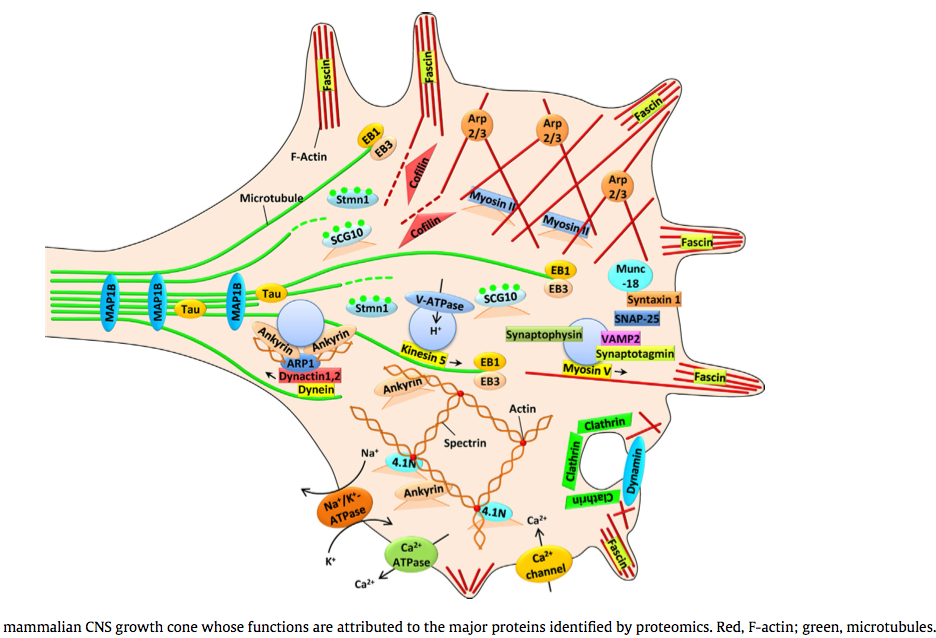
(2014) Proteomic identification of the molecular basis of mammalian CNS growth cones *
The trip of the tip: understanding the growth cone machinery *
^ looks like disabling RhoA doesn't encourage axon growth outside of the brain
Shrooms
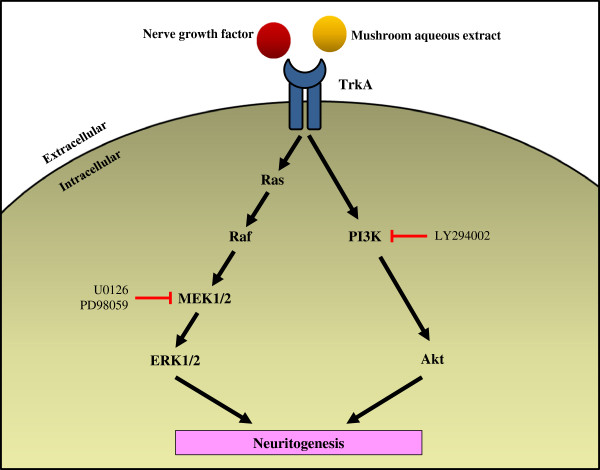
Potentiation of neuritogenic activity of medicinal mushrooms in rat pheochromocytoma cells
- Ganoderma lucidum, G. neo-japonicum and G. frondosa may contain NGF-like bioactive compound(s) for maintaining and regenerating the neuronal communications network. The present study reports the first evidence of the neuritogenic effects of aqueous extracts of basidiocarps of G. neo-japonicum in-vitro and showed the involvement of MEK/ERK1/2 and P13K/Akt signaling pathways for neuritogenesis in PC-12 cells.
- G. lucidum, L. rhinocerotis, P. giganteus, G. frondosa and C. militaris may be developed as safe and healthy dietary supplements for brain and cognitive health
DIHEXA
Ashwagandha
Axon (or dendrite) predominant outgrowth induced by constituents from Ashwagandha *
- "results suggest that axons are predominantly extended by withanolide A, and dendrites by withanosides IV and VI."
- withanoside IV a.k.a. Sominone = GDNF-independent stimulator of the RET pathway and/or a novel modulator of RET signalling
[2]