Neuroscience
Neuro
- CPEB regulates rate of mRNA -> protein translation. Hence high CPEB levels in active synapse will lead to higher protein synthesis there.
Axonal protein synthesis and the regulation of local mitochondrial function
- creb1 expression must be sustained for a relatively long time to support the consolidation of LTF.
- VPA may increase creb1 expression
http://www.ncbi.nlm.nih.gov/pubmed/?term=hdac+inhibitor+memory
(2012) The molecular biology of memory: cAMP, PKA, CRE, CREB-1, CREB-2, and CPEB
- (2003) CREB, memory enhancement and the treatment of memory disorders: promises, pitfalls and prospects
- However, CREB and its close relatives cAMP response element modulator and activating transcription factor-1 are ubiquitous proteins with several critical functions. This creates hurdles that the authors believe may limit the usefulness of CREB per se as a target for the development of memory-enhancing drugs, and focus on components of the upstream signalling pathways or on specific downstream targets will be required.
- (1995) Aplysia CREB2 represses long-term facilitation: relief of repression converts transient facilitation into long-term functional and structural change
- The switch from short- to long-term facilitation induced by behavioral sensitization in Aplysia involves CREB-like proteins, as well as the immediate-early gene ApC/EBP. Using the bZIP domain of ApC/EBP in a two-hybrid system, we have cloned ApCREB2, a transcription factor constitutively expressed in sensory neurons that resembles human CREB2 and mouse ATF4. ApCREB2 represses ApCREB1-mediated transcription in F9 cells. Injection of anti-ApCREB2 antibodies into Aplysia sensory neurons causes a single pulse of serotonin (5-HT), which induces only short-term facilitation lasting minutes, to evoke facilitation lasting more than 1 day. This facilitation has the properties of long-term facilitation: it requires transcription and translation, induces the growth of new synaptic connections, and occludes further facilitation by five pulses of 5-HT.
- So, knock out CREB2 for better L-LTP
- The switch from short- to long-term facilitation induced by behavioral sensitization in Aplysia involves CREB-like proteins, as well as the immediate-early gene ApC/EBP. Using the bZIP domain of ApC/EBP in a two-hybrid system, we have cloned ApCREB2, a transcription factor constitutively expressed in sensory neurons that resembles human CREB2 and mouse ATF4. ApCREB2 represses ApCREB1-mediated transcription in F9 cells. Injection of anti-ApCREB2 antibodies into Aplysia sensory neurons causes a single pulse of serotonin (5-HT), which induces only short-term facilitation lasting minutes, to evoke facilitation lasting more than 1 day. This facilitation has the properties of long-term facilitation: it requires transcription and translation, induces the growth of new synaptic connections, and occludes further facilitation by five pulses of 5-HT.
- (1999) A transient, neuron-wide form of CREB-mediated long-term facilitation can be stabilized at specific synapses by local protein synthesis
- In a culture system where a bifurcated Aplysia sensory neuron makes synapses with two motor neurons, repeated application of serotonin (5-HT) to one synapse produces a CREB-mediated, synapse-specific, long-term facilitation, which can be captured at the opposite synapse by a single pulse of 5-HT. Repeated pulses of 5-HT applied to the cell body of the sensory neuron produce a CREB-dependent, cell-wide facilitation, which, unlike synapse-specific facilitation, is not associated with growth and does not persist beyond 48 hr. Persistent facilitation and synapse-specific growth can be induced by a single pulse of 5-HT applied to a peripheral synapse. Thus, the short-term process initiated by a single pulse of 5-HT serves not only to produce transient facilitation, but also to mark and stabilize any synapse of the neuron for long-term facilitation by means of a covalent mark and rapamycin-sensitive local protein synthesis.
- (2005) Epigenetic mechanisms in memory formation
- Discoveries concerning the molecular mechanisms of cell differentiation and development have dictated the definition of a new sub-discipline of genetics known as epigenetics. Epigenetics refers to a set of self-perpetuating, post-translational modifications of DNA and nuclear proteins that produce lasting alterations in chromatin structure as a direct consequence, and lasting alterations in patterns of gene expression as an indirect consequence. The area of epigenetics is a burgeoning subfield of genetics in which there is considerable enthusiasm driving new discoveries. Neurobiologists have only recently begun to investigate the possible roles of epigenetic mechanisms in behaviour, physiology and neuropathology. Strikingly, the relevant data from the few extant neurobiology-related studies have already indicated a theme - epigenetic mechanisms probably have an important role in synaptic plasticity and memory formation.
- (2005) Chromatin remodeling in neural development and plasticity
- Neural stem cells generate distinct cell types for tissue formation and cell replacement during development and throughout adulthood. Neural development and plasticity are determined by both extrinsic and intrinsic factors that interface to regulate gene programs for controlling neuronal cell fate and function. Recent reports have shown that chromatin remodeling and epigenetic gene regulation play an important role in such diverse areas as neural cell fate specification and synaptic development and function. These epigenetic mechanisms include:
- cell-type-specific transcriptional regulators
- histone modifications and chromatin remodeling enzymes,
- and the activity of retrotransposons.
- Neural stem cells generate distinct cell types for tissue formation and cell replacement during development and throughout adulthood. Neural development and plasticity are determined by both extrinsic and intrinsic factors that interface to regulate gene programs for controlling neuronal cell fate and function. Recent reports have shown that chromatin remodeling and epigenetic gene regulation play an important role in such diverse areas as neural cell fate specification and synaptic development and function. These epigenetic mechanisms include:
- (1997) Synaptic tagging and long-term potentiation
- Repeated stimulation of hippocampal neurons can induce an immediate and prolonged increase in synaptic strength that is called long-term potentiation (LTP)-the primary cellular model of memory in the mammalian brain. An early phase of LTP (lasting less than three hours) can be dissociated from late-phase LTP by using inhibitors of transcription and translation, Because protein synthesis occurs mainly in the cell body, whereas LTP is input-specific, the question arises of how the synapse specificity of late LTP is achieved without elaborate intracellular protein trafficking. We propose that LTP initiates the creation of a short-lasting protein-synthesis-independent 'synaptic tag' at the potentiated synapse which sequesters the relevant protein(s) to establish late LTP. In support of this idea, we now show that weak tetanic stimulation, which ordinarily leads only to early LTP, or repeated tetanization in the presence of protein-synthesis inhibitors, each results in protein-synthesis-dependent late LTP, provided repeated tetanization has already been applied at another input to the same population of neurons. The synaptic tag decays in less than three hours. These findings indicate that the persistence of LTP depends not only on local events during its induction, but also on the prior activity of the neuron.
- (2004) Synapse to nucleus signaling during long-term synaptic plasticity; a role for the classical active nuclear import pathway
- The requirement for transcription during long-lasting plasticity indicates that signals generated at the synapse must be transported to the nucleus. We have investigated whether the classical active nuclear import pathway mediates intracellular retrograde signal transport in Aplysia sensory neurons and rodent hippocampal neurons. We found that importins localize to distal neuronal processes, including synaptic compartments, where they are well positioned to mediate synapse to nucleus signaling. In Aplysia, stimuli known to produce long-lasting but not short-lasting facilitation triggered importin nuclear translocation. In hippocampal neurons, NMDA receptor activation but not depolarization induced importin nuclear translocation. We further showed that LTP-inducing stimuli recruited active nuclear import in hippocampal slices. Together with our finding that long-term facilitation of Aplysia sensory-motor synapses required active nuclear import, our results indicate that regulation of the active nuclear import pathway plays a critical role in transporting synaptically generated signals into the nucleus during learning-related forms of plasticity.
- (1989) An essential role for postsynaptic calmodulin and protein kinase activity in long-term potentiation
- The phenomenon of long-term potentiation (LTP), a long lasting increase in the strength of synaptic transmission which is due to brief, repetitive activation of excitatory afferent fibres, is one of the most striking examples of synaptic plasticity in the mammalian brain. In the CA1 region of the hippocampus, the induction of LTP requires activation of NMDA (N-methyl-D-aspartate) receptors by synaptically released glutamate with concomitant postsynaptic membrane depolarization. This relieves the voltage-dependent magnesium block of the NMDA-receptor ion channel, allowing calcium to flow into the dendritic spine. Although calcium has been shown to be a necessary trigger for LTP (refs 11, 12), little is known about the immediate biochemical processes that are activated by calcium and are responsible for LTP. The most attractive candidates have been calcium/calmodulin-dependent protein kinase II (CaM-KII) (refs 13-16), protein kinase C (refs 17-19), and the calcium-dependent protease, calpain. Extracellular application of protein kinase inhibitors to the hippocampal slice preparation blocks the induction of LTP (refs 21-23) but it is unclear whether this is due to a pre- and/or postsynaptic action. We have found that intracellular injection into CA1 pyramidal cells of the protein kinase inhibitor H-7, or of the calmodulin antagonist calmidazolium, blocks LTP. Furthermore, LTP is blocked by the injection of synthetic peptides that are potent calmodulin antagonists and inhibit CaM-KII auto- and substrate phosphorylation. These findings demonstrate that in the postsynaptic cell both activation of calmodulin and kinase activity are required for the generation of LTP, and focus further attention on the potential role of CaM-KII in LTP.
- ^ same as (1989) Inhibition of postsynaptic PKC or CaMKII blocks induction but not expression of LTP
- (1988) Persistent protein kinase activity underlying long-term potentiation
- Long-term potentiation (LTP) of synaptic transmission in the hippocampus is a much-studied example of synaptic plasticity. Although the role of N-methyl-D-aspartate (NMDA) receptors in the induction of LTP is well established, the nature of the persistent signal underlying this synaptic enhancement is unclear. Involvement of protein phosphorylation in LTP has been widely proposed, with protein kinase C (PKC) and calcium-calmodulin kinase type II (CaMKII) as leading candidates. Here we test whether the persistent signal in LTP is an enduring phosphoester bond, a long-lived kinase activator, or a constitutively active protein kinase by using H-7, which inhibits activated protein kinases and sphingosine, which competes with activators of PKC (ref. 17) and CaMKII (ref. 18). H-7 suppressed established LTP, indicating that the synaptic potentiation is sustained by persistent protein kinase activity rather than a stably phosphorylated substrate. In contrast, sphingosine did not inhibit established LTP, although it was effective when applied before tetanic stimulation. This suggests that persistent kinase activity is not maintained by a long-lived activator, but is effectively constitutive. Surprisingly, the H-7 block of LTP was reversible; evidently, the kinase directly underlying LTP remains activated even though its catalytic activity is interrupted indicating that such kinase activity does not sustain itself simply through continual autophosphorylation (see refs 9, 13, 15).
- (1999) Rapid spine delivery and redistribution of AMPA receptors after synaptic NMDA receptor activation
- To monitor changes in alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA) receptor distribution in living neurons, the AMPA receptor subunit GluR1 was tagged with green fluorescent protein (GFP). This protein (GluR1-GFP) was functional and was transiently expressed in hippocampal CA1 neurons. In dendrites visualized with two-photon laser scanning microscopy or electron microscopy, most of the GluR1-GFP was intracellular, mimicking endogenous GluR1 distribution. Tetanic synaptic stimulation induced a rapid delivery of tagged receptors into dendritic spines as well as clusters in dendrites. These postsynaptic trafficking events required synaptic N-methyl-D-aspartate (NMDA) receptor activation and may contribute to the enhanced AMPA receptor-mediatedtransmission observed during long-term potentiation and activity-dependent synaptic maturation.
- (2009) PKMzeta maintains spatial, instrumental, and classically conditioned long-term memories
- How long-term memories are stored is a fundamental question in neuroscience. The first molecular mechanism for long-term memory storage in the brain was recently identified as the persistent action of protein kinase Mzeta (PKMzeta)...
- (2007) Neuronal competition and selection during memory formation
- Competition between neurons is necessary for refining neural circuits during development and may be important for selecting the neurons that participate in encoding memories in the adult brain. To examine neuronal competition during memory formation, we conducted experiments with mice in which we manipulated the function of CREB (adenosine 3',5'-monophosphate response element-binding protein) in subsets of neurons. Changes in CREB function influenced the probability that individual lateral amygdala neurons were recruited into a fear memory trace. Our results suggest a competitive model underlying memory formation, in which eligible neurons are selected to participate in amemorytrace as a function of their relative CREB activity at the time of learning.
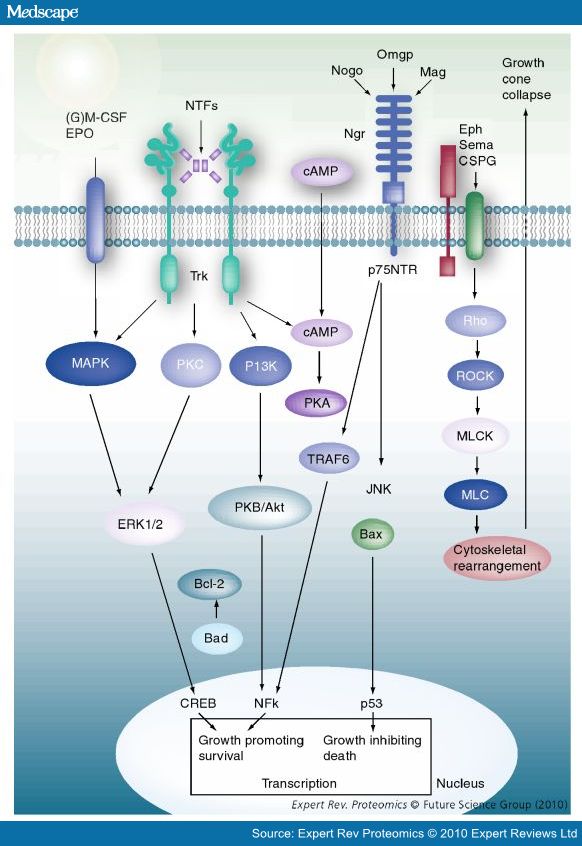
Nogo
(2002) Nogo-66 receptor antagonist peptide promotes axonal regeneration
- The Nogo-66(1 40) antagonist peptide (NEP1 40) blocks Nogo-66 (i.e. CNS myelin inhibition of axonal outgrowth) in vitro
(2002) Truncated Soluble Nogo Receptor Binds Nogo-66 and Blocks Inhibition of Axon Growth by Myelin
- This analysis has led to the identification of a soluble, truncated NgR (NgREcto) that antagonizes the neurite outgrowth-inhibitory effects of Nogo
(2003) Targeting the Nogo receptor to treat central nervous system injuries *
- Strittmatter lays out the scene in 2002, note ROCK inhibitor Fasudil
- Other therapeutic candidates and targets have been assessed for a potential role in CNS axonal regeneration, including neurotrophic factors, such as a NGF, brain-derived neurotrophic factor (BDNF) and neuro- trophin-3 (NT-3), inosine, neuroimmunophilins, chondroitinase ABC and cyclic AMP/protein kinase A (cAMP/PKA).
- Neuroimmunophilins -- FK506 <-- TODO: check this
- NgR(310)ecto (intrathical)
- Comparison with other NgR antagonists <-- shows that blocking Nogo66 is sufficient, no need to also block MAG&OMgp
- The recent determination of the structure of the NgR(310)ecto ligand-binding domain (Barton et al., 2003; He et al., 2003) could potentially facilitate the rational design of small molecule NgR antagonists.
- Pharmacological methods to perturb the CNS myelin inhibitor system have included:
- anti-Nogo antibodies (Schnell and Schwab, 1990; Bregman et al., 1995),
- a NgR antagonist peptide (GrandPre et al., 2002; Li and Strittmatter, 2003),
- Rho pathway inhibitors (Dergham et al., 2002; Fournier et al., 2003),
- and cAMP elevation (Neumann et al., 2002; Qiu et al., 2002; Nikulina et al., 2004; Pearse et al., 2004).
- All four methods have some efficacy in vivo. The first two approaches are specific for Nogo and do not disrupt MAG or OMgp action. Blockade of Rho signaling blocks myelin and glial scar inhibition but may modify many other forms of cellular motility. Alterations of cAMP signaling may also have pleiotropic effects on numerous signaling pathways.
(2005)Nogo-A antibody improves regeneration and locomotion of spinal cord-injured rats
*
- studying the effects of two new monospecific monoclonal antibodies directed against defined regions of Nogo-A, the antibodies 11C7 and 7B12
- acutely applied for 2 weeks as highly purified IgGs into the subdural space over the spinal cord by fine catheters connected to osmotic minipumps, a procedure that can be applied to higher mammals and also humans.
(2006) Delayed Nogo receptor therapy improves recovery from spinal cord contusion
- Myelin-derived Nogo-A protein limits axonal growth after CNS injury. One domain binds to the Nogo-66 receptor to inhibit axonal outgrowth, whereas a second domain, Amino-Nogo, inhibits axonal outgrowth and cell adhesion through unknown mechanisms. Here, we show that Amino-Nogo inhibition depends strictly on the composition of the extracellular matrix, suggesting that Amino-Nogo inhibits the function of certain integrins. Amino-Nogo inhibition can be partially overcome by antibodies that activate integrin β1 or by the addition of Mn2+, an integrin activator.
- administration of [sNgR --] a soluble peptide fragment of the NgR that binds to and blocks all three NgR ligands can promote regeneration
- Knocking down neuronal Nogo-A by small interfering RNA results in a marked increase of neurite outgrowth. We constructed a nonreplicating herpes simplex virus vector (QHNgSR) to express a truncated soluble fragment of Nogo receptor 1 (NgSR). NgSR released from QHNgSR prevented myelin inhibition of neurite extension by hippocampal and DRG neurons in vitro. NgSR prevents RhoA activation by myelin and decreases neuronal Nogo-A.
- [...]triple therapy combining NgR1 decoy, ChABC and preconditioning, allows axons to regenerate millimeters past the spinal cord injury site. The benefit of a pre-conditioning injury is most robust, but a peripheral nerve injury coincident with, or 3 days after, spinal cord injury also synergizes with NgR1 decoy and ChABC. Thus, maximal axonal regeneration and neural repair is achieved by combining independently effective pharmacological approaches.
(2013) Anatomical Plasticity of Adult Brain is Titrated by Nogo Receptor 1
- Several ligands for NgR1 have been described, including Nogo-A, MAG and OMgp from oligodendrocytes, [CSPGs] and LGI1 (Thomas et al., 2010).
- While Nogo-A/B is required, MAG, OMgp, CSPGs and LGI1 are alternate NgR1 ligands (Dickendesher et al., 2012; Liu et al., 2002; Thomas et al., 2010; Wang et al., 2002a) that may have co-essential roles to limit synaptic rearrangement in the cerebral cortex. CSPGs are known to participate in electrophysiological plasticity of the cerebral cortex (Pizzorusso et al., 2002). The alternate Nogo receptor, PirB, has also been implicated in experience dependent plasticity
LongeCity: Restoring critical period plasticity through deactivating axon growth inhibitor
PATENT 2009: Axon Regeneration with PKC Inhibitors
TODO:
- CREB is a cellular transcription factor. It binds to certain DNA sequences called cAMP response elements (CRE), thereby increasing or decreasing the transcription of the downstream genes.
- Genes whose transcription is regulated by CREB include: c-fos, the neurotrophin BDNF (brain-derived neurotrophic factor), tyrosine hydroxylase, and many neuropeptides (such as somatostatin, enkephalin, VGF, and corticotropin-releasing hormone).
- "CREB has a well-documented role in neuronal plasticity and long-term memory formation in the brain. CREB has been shown to be integral in the formation of spatial memory."
http://www.ncbi.nlm.nih.gov/pubmed/16476423
http://www.sabiosciences.com/rt_pcr_product/HTML/PAHS-126Z.html ? http://journal.frontiersin.org/Journal/10.3389/neuro.02.001.2010/full ?
Dynamic epigenetic regulation in neurons *
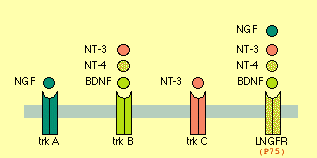
- Neurotrophin = {NGF, BNDF, NT3, NT4}
- Neurotrophic Factor = Neurotrophin + {GDNF, CNTF}.
NGF -- promotes Axon growth (branching and a bit of elongation)
BNDF -- CNS&PNS, supports survival of existing neurons, growth & diff'n of new neurons & synapses. DiHexa = 10M * BNDF. Binds to TrkB and P75 (a.k.a. L-NGFr) receptors.
Functional role of neurotrophin-3 in synapse regeneration by spiral ganglion neurons on inner hair cells after excitotoxic trauma in vitro <-- NT3 vital for restoring ear-hair-cell-neurons (TINNITUS?).
GSK3 -- can't quite fit this anywhere else, maybe relevant maybe not
Role of GSK3 signaling in neuronal morphogenesis
GSK3 signalling in neural development
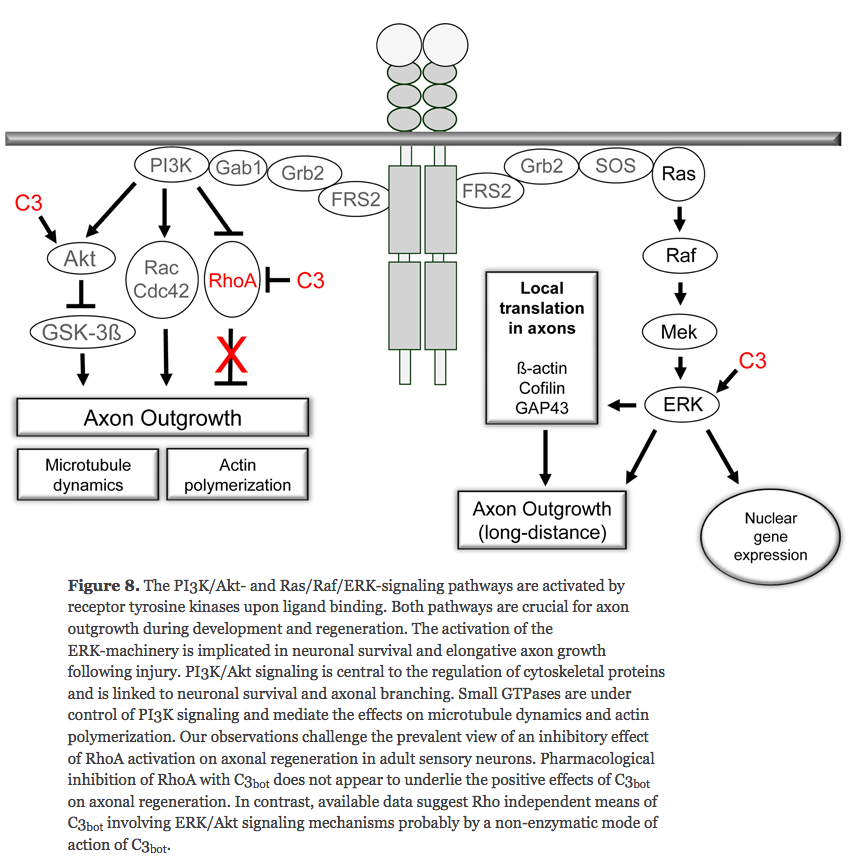
Roles of PI3K/AKT/GSK3/mTOR Pathway in Cell Signaling of Mental Illnesses
Axon Growth-Stimulus Package Includes Local Translation
- Translation of localized mRNAs is an important mechanism for controlling spatially discrete cellular processes. The polarity protein Par-3 is locally translated in axons in response to factors such as NGF and netrin-1, and this increased expression is necessary for factor-stimulated axonal outgrowth.
- When and NGF bead lands on an axon, a new growth cone is induced at the contact site, and a branch sprouts from the axon.
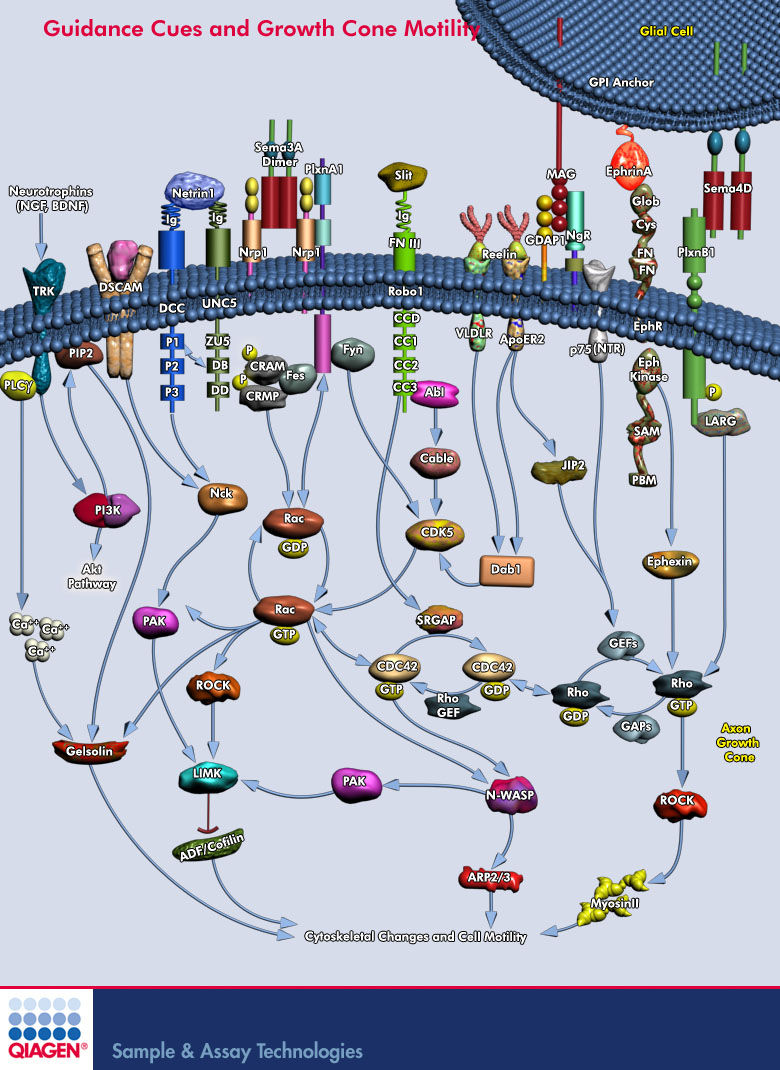
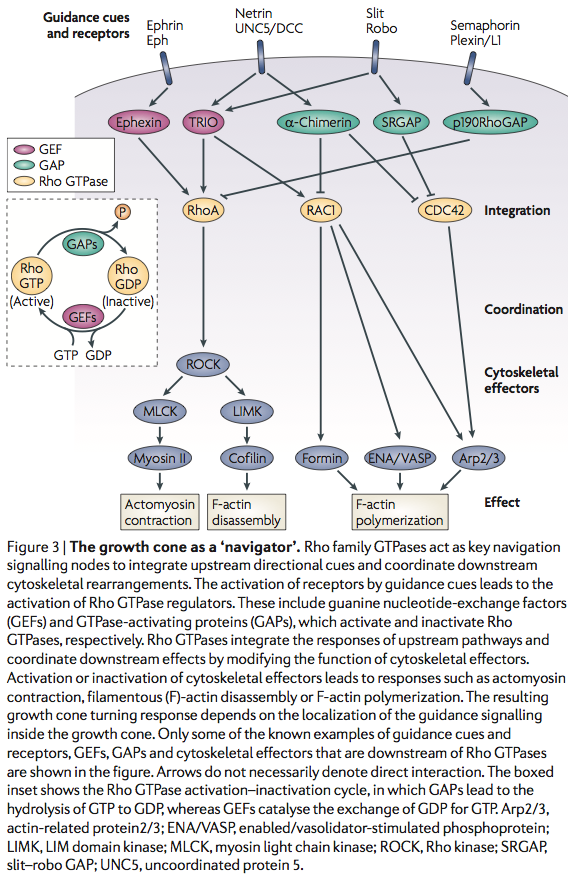
Lamellipodium <-- 'Rac promotes lamellipodia while cdc42 promotes filopodia' (filopodia are the fingers, the probes on the growthcone and lamellipodium is the webbing)
http://en.wikipedia.org/wiki/Semaphorin <-- Semaphorins are a class of secreted and membrane proteins that act as axonal growth cone guidance molecules. [...] The main receptors for semaphorins are plexins, which have established roles in regulating Rho-family GTPases. Recent work shows that plexins can also influence R-Ras, which, in turn, can regulate integrins.
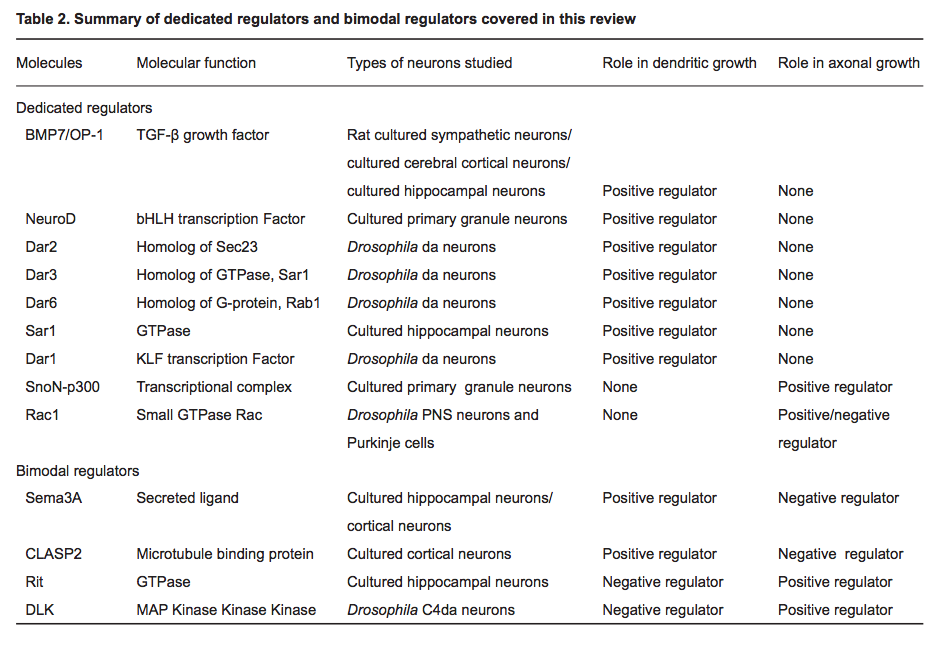
(2014) Regulatory mechanisms underlying the differential growth of dendrites and axons *
- "Recent studies have uncovered two distinct types of regulatory mechanisms that differentiate dendritic and axonal growth: dedicated mechanisms and bimodal mechanisms. Dedicated mechanisms regulate either dendrite- specific or axon-specific growth; in contrast, bimodal mechanisms direct dendritic and axonal development in opposite manners."
The Novel GTPase Rit Differentially Regulates Axonal and Dendritic Growth
Sema3A is where?
Sema3A -> CRMP -> Rac/GDP -> Rac/GTP -> CDC42 -> Rho -> ROCK -> myosinll
(2010) Deciphering Proteins and their Functions in the Regenerating Retina
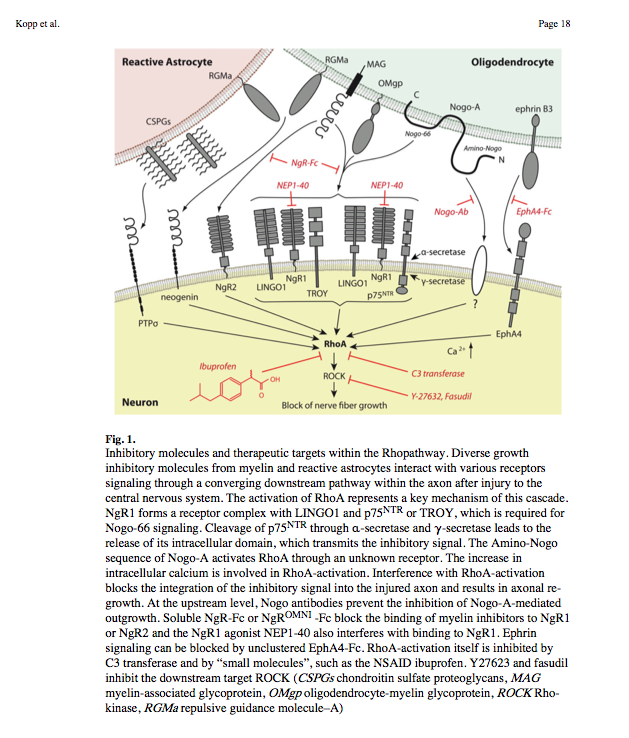
- Nogo-A, -B and -C all contain a 66-amino acid extracellular domain (Nogo-66) that alone can inhibit neurite outgrowth and induce growth-cone collapse.
- Remarkably, a single neuronal protein (NgR), binds Nogo, MAG and OMgp.[101] NgR is thought to form a complex with its coreceptors (p75NTR, TROY, a member of the TNF receptor family, and Lingo-1), thereby transducing a myelin/NgR signal as the inhibitory signal to the cell interior[95] and potentially activating the small GTPase RhoA (Figure 3B). Another myelin-associated neurite outgrowth inhibitor, the RGM, which activates the Rho/ROCK signaling cascade independently of the NgR pathway, has recently been discovered. Consistent with these findings, deleting oligodendrocytes, myelin or Schwann cell-derived factor-induced cleavage of NgR and Nogo-A, and inactivation of p75NTR [105] enhances the regeneration of descending visual pathways. Consistent with their inhibitory effects on neurite outgrowth, approaches counteracting NgR, Nogo-A or RhoA have been shown to significantly enhance long-distance axon regeneration in animal models of acute retinal injury.
- ^ need to check up on RGM! Looks as though disabling NgR is not sufficient. also need to disable RGM as either one can activate Rho -> ROCK.
- (Wait: The therapeutic effects of Rho-ROCK inhibitors on CNS disorders <-- says 'In the adult CNS, injured axons regenerate poorly due to the presence of myelin-associated axonal growth inhibitors such as myelin-associated glycoprotein (MAG), Nogo, oligodendrocyte-myelin glycoprotein (OMgp), and the recently identified repulsive guidance molecule (RGM). The effects of these inhibitors are reversed by blockade of the Rho-ROCK pathway in vitro, and the inhibition of this pathway promotes axonal regeneration and functional recovery in the injured CNS in vivo.')
So how about directly disable Rho using Ibuprofen:
Small-molecule-induced Rho-inhibition: NSAIDs after spinal cord injury
(2005) Local translation of RhoA regulates growth cone collapse
- Neuronal development requires highly coordinated regulation of the cytoskeleton within the developing axon. This dynamic regulation manifests itself in axonal branching, turning and pathfinding, presynaptic differentiation, and growth cone collapse and extension. Semaphorin 3A (Sema3A), a secreted guidance cue that primarily functions to repel axons from inappropriate targets, induces cytoskeletal rearrangements that result in growth cone collapse1. These effects require intra-axonal messenger RNA translation. Here we show that transcripts for RhoA, a small guanosine triphosphatase (GTPase) that regulates the actin cytoskeleton, are localized to developing axons and growth cones, and this localization is mediated by an axonal targeting element located in the RhoA 3' untranslated region (UTR). Sema3A induces intra-axonal translation of RhoA mRNA, and this local translation of RhoA is necessary and sufficient for Sema3A-mediated growth cone collapse. These studies indicate that local RhoA translation regulates the neuronal cytoskeleton and identify a new mechanism for the regulation of RhoA signalling.
Intra-Axonal Translation of RhoA Promotes Axon GrowthInhibition by CSPG
- RhoA transcripts have been detected in axons of embryonic dorsal root ganglia (DRG), retinal ganglion, and cortical and hippocampal neurons, and are locally translated in DRG axons in response to the inhibitory guidance cue Semaphorin 3A (Sema3A) and mediate growth cone collapse (Wu et al., 2005).
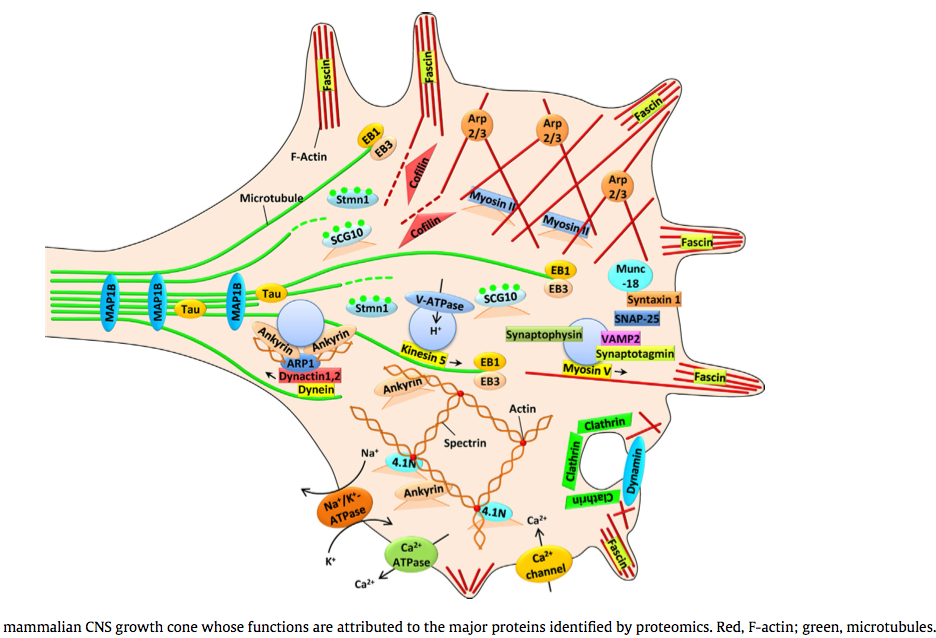
(2014) Proteomic identification of the molecular basis of mammalian CNS growth cones *
The trip of the tip: understanding the growth cone machinery *
^ looks like disabling RhoA doesn't encourage axon growth outside of the brain
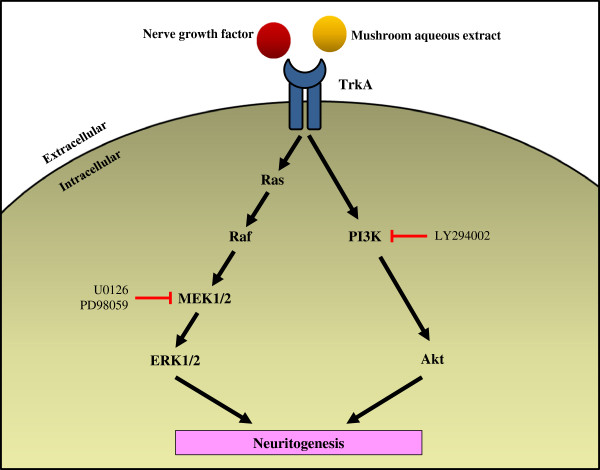
Shrooms
Potentiation of neuritogenic activity of medicinal mushrooms in rat pheochromocytoma cells
- Ganoderma lucidum, G. neo-japonicum and G. frondosa may contain NGF-like bioactive compound(s) for maintaining and regenerating the neuronal communications network. The present study reports the first evidence of the neuritogenic effects of aqueous extracts of basidiocarps of G. neo-japonicum in-vitro and showed the involvement of MEK/ERK1/2 and P13K/Akt signaling pathways for neuritogenesis in PC-12 cells.
- G. lucidum, L. rhinocerotis, P. giganteus, G. frondosa and C. militaris may be developed as safe and healthy dietary supplements for brain and cognitive health
DIHEXA
Cellular mechanisms underlying the regulation of dendritic development by hepatocyte growth factor
- "regulation of cortical dendritic morphology by HGF is mediated by activation of the MAPK pathway, phosphorylation of CREB and interaction of CREB with CRTC1"
- "HGF stimulates Ca2+ influx into dendrites through the NMDA receptor and that this effect is necessary for the changes in dendritic morphology induced by HGF"
HGF regulates the development of cortical pyramidal dendrites
Media:(2009)_Hepatocyte_Growth_Factor_in_Synaptic_Plasticity_and_Alzheimer’s_Disease.pdf
Ashwagandha
Axon (or dendrite) predominant outgrowth induced by constituents from Ashwagandha *
- "results suggest that axons are predominantly extended by withanolide A, and dendrites by withanosides IV and VI."
- withanoside IV a.k.a. Sominone = GDNF-independent stimulator of the RET pathway and/or a novel modulator of RET signalling
[4]